For the projects in which we are generating novel protein/protein interactions, an inverse approach is used to further understand the biophysical factors that drive protein/protein interactions. The approach is considered inverse because, instead of analyzing examples of natural protein complexes, which has been pursued extensively by scientists such as Professor Janet Thornton, we strive to develop a deeper understanding of the factors that drive biomolecular association by attempting to drive complex formation of previously monomeric proteins. Stated otherwise, we re-engineer monomeric proteins to drive them to dimerize into specific structures.
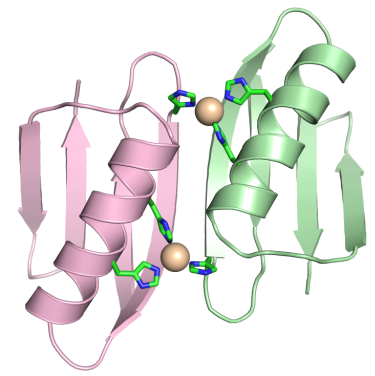
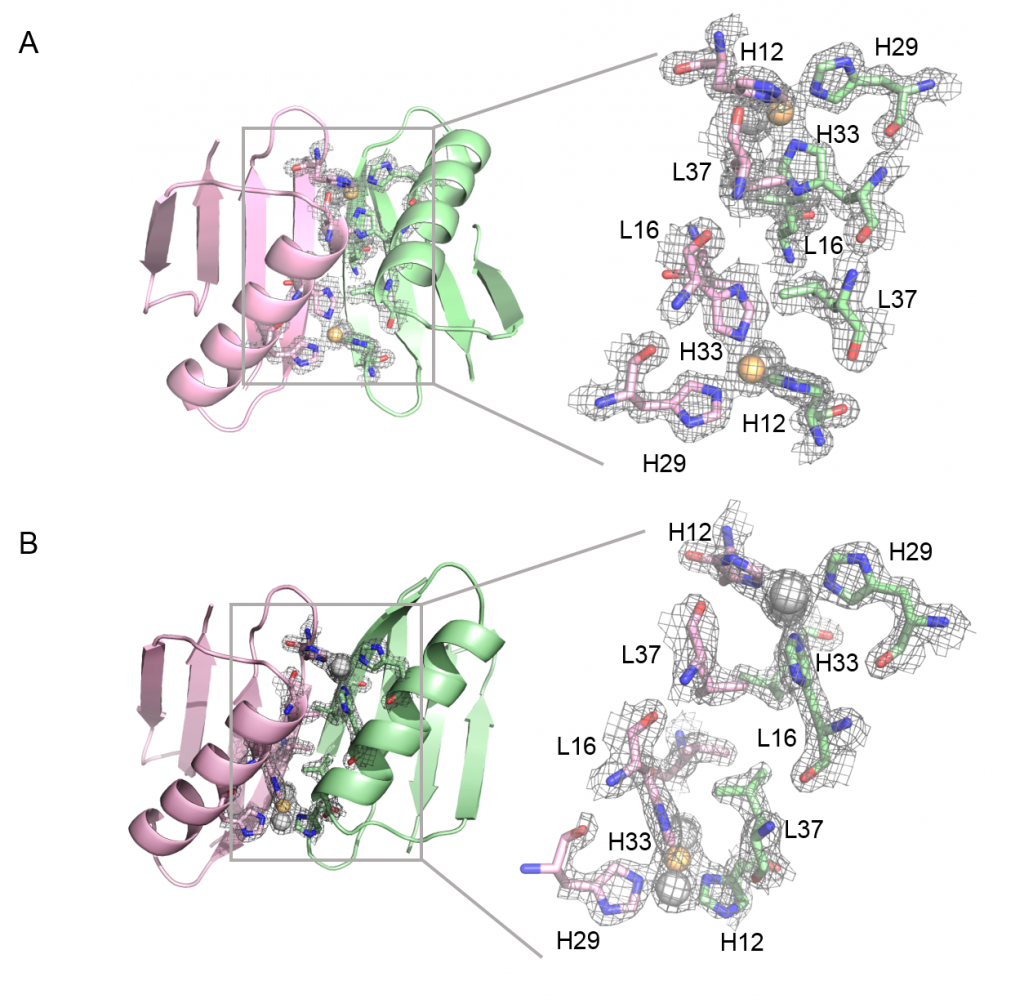
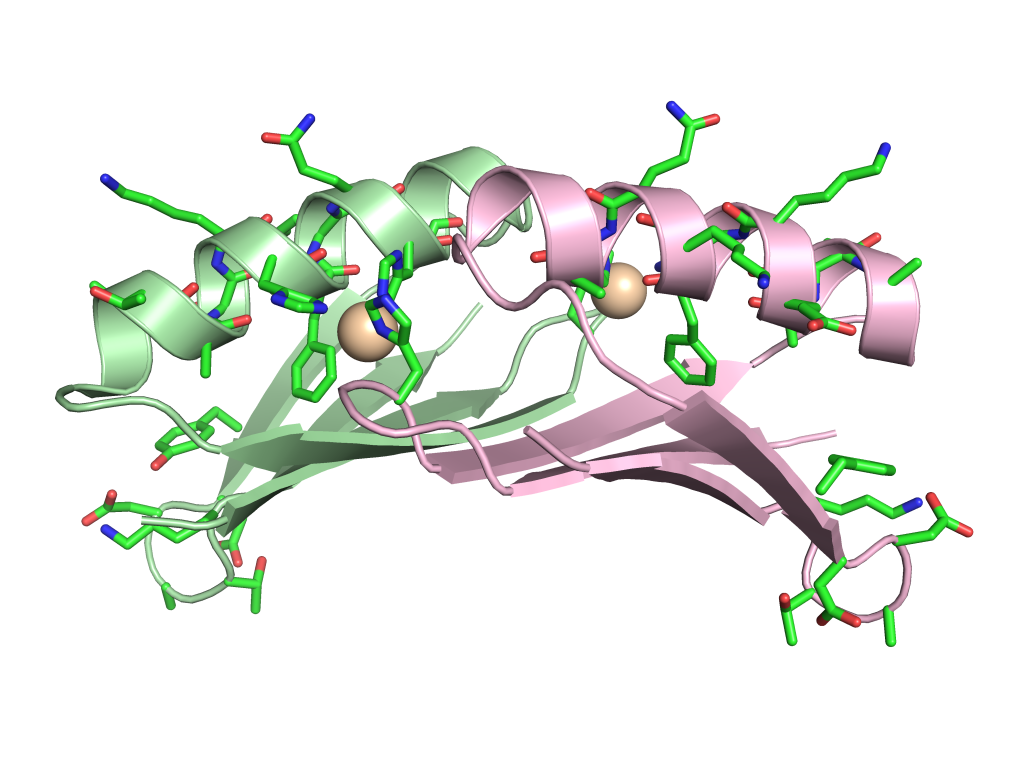
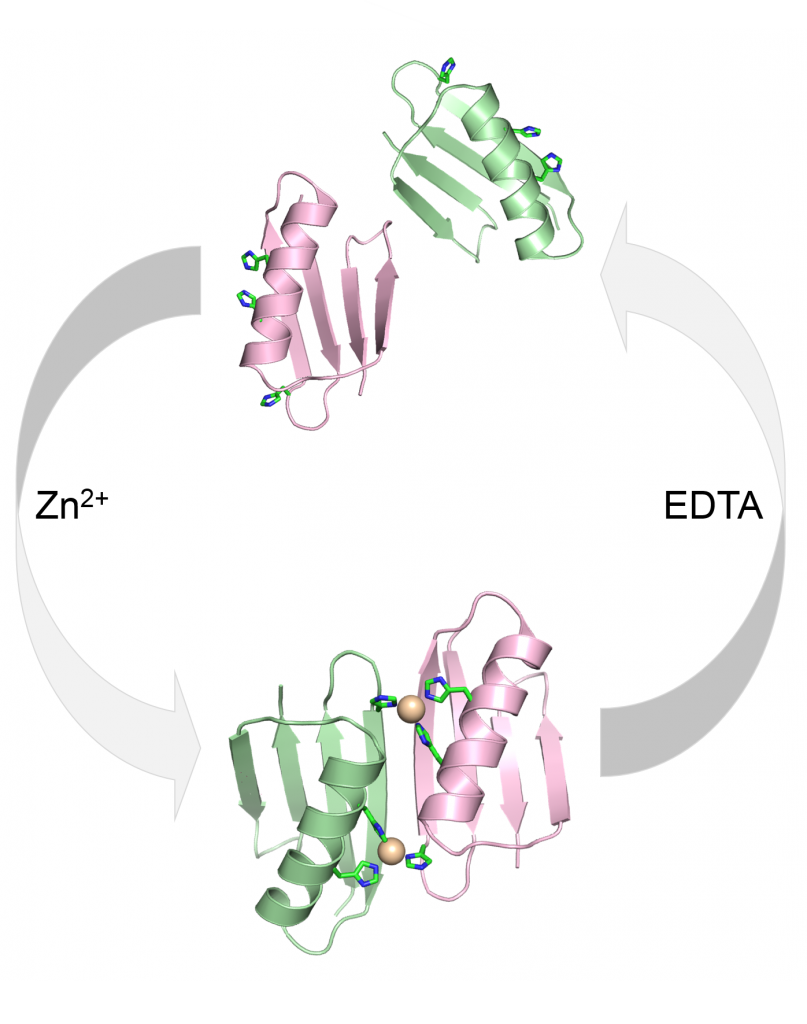
Metal-Controlled Protein Dimers. A current focus of the of these efforts is the design of specific protein/protein interactions starting with a protein scaffold that is completely monomeric under standard condition (i.e., protein-G). We recently had significant success in the design of three metal-mediated homodimer proteins. The three designed proteins are fully monomeric in the absence of metal yet form high affinity homodimers in the presence of zinc sulfate. In fact, the complex of highest affinity has a binding constant that less than 75 pmol as determined by fluorescence-based analytical ultracentrifugation. This level of tight binding and high specificity is unprecedented in the field of protein design.
Maniaci, B., Lipper, C. H., Anipindi, D. L., Erlandsen, H., Cole, J. L., Stec, B., Huxford, T., Love, J. J., Design of High-Affinity Metal-Controlled Protein Dimers. Biochemistry, 2019, 58, 2199-2207. doi: 10.1021/acs.biochem.9b00055.
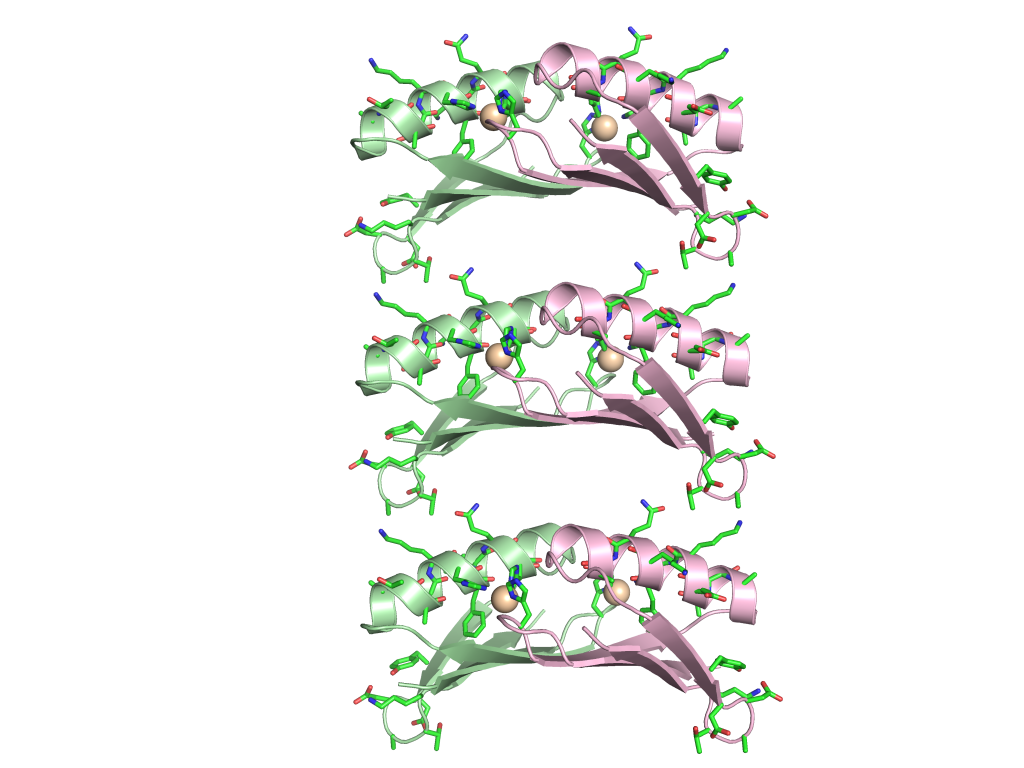
Current and Future Focus: We are currently using these findings to build novel biomaterials by fusing passenger proteins to the metal binding proteins. This will allow for transient association and dissociation of passenger proteins driven by the addition of metal or chelating agent such as EDTA. We are also working to incorporate the metal-mediated homodimer into novel biomaterials in which assembly can be controlled via the selective addition of specific metal atoms (images below).